Endocrinopatias múltiplas adquiridas pelo uso de um inibidor de checkpoint - Pembrolizumab: Um Relato de Caso
Conteúdo do artigo principal
Resumo
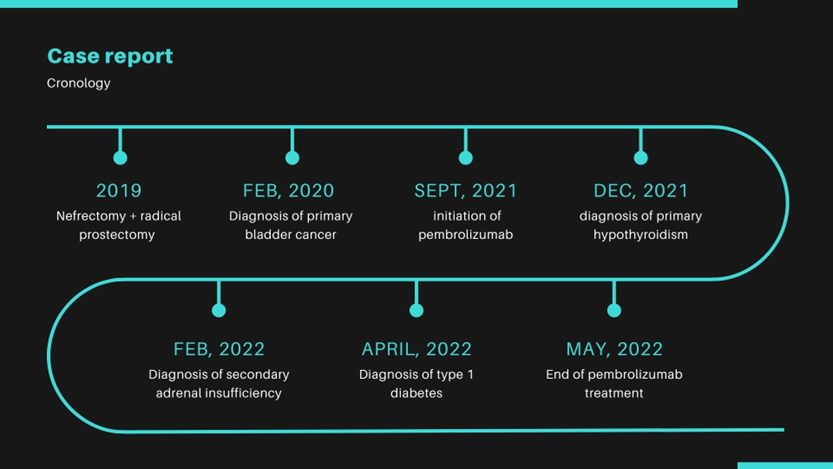
A imunoterapia com inibidores de checkpoint (ICIs) tem sido cada vez mais empregada no tratamento de muitas neoplasias malignas, e as endocrinopatias são um dos efeitos colaterais mais frequentes. Este relato de caso descreve um homem de 70 anos que enfrentou várias endocrinopatias (hipotireoidismo primário, insuficiência adrenal secundária e diabetes mellitus) após o início da imunoterapia com um ICI (pembrolizumabe) para o tratamento de câncer de bexiga urotelial. Discutimos a prevalência, fisiopatologia, triagem e diagnóstico de cada uma dessas anomalias. Os clínicos precisam estar cientes dessas complicações endócrinas da terapia com ICIs e estar preparados para o diagnóstico precoce e manejo adequado.
Detalhes do artigo

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Authors retain the copyright of their articles and grant the journal the right of first publication under the Creative Commons Attribution (CC BY) license, which allows others to share and adapt the work with proper attribution.
Referências
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ. 2018;360.
Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ. 2018;363.
Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016 Nov;12(11):2777-2789. doi: 10.1080/21645515.2016.1199310. Epub 2016 Jul 11. PMID: 27398650; PMCID: PMC5137544
Figueroa-Perez N, Kashyap R, Bal D, et al. (August 06, 2021) Autoimmune Myasthenia, Primary Adrenal Insufficiency, and Progressive Hypothyroidism Due to Pembrolizumab and Axitinib Combination Regimen. Cureus 13(8): e16933. doi:10.7759/cureus.16933
Hattersley R, Nana M, Lansdown AJ. Endocrine complications of immunotherapies: a review. Clin Med (Lond). 2021 Mar;21(2):e212-e222. doi: 10.7861/clinmed.2020-0827
Sankar K, Macfarlane M, Cooper O, et al. Pembrolizumab-induced diabetic ketoacidosis: A review of critical care case. Cureus. 2021;13(10).
Ayesh H, Burmeister C, Tomcho JC, Fatima R, Hejeebu SK. Pembrolizumab-induced thyroiditis. Am J Ther. 2023;30(3). doi: 10.1097/MJT.0000000000001367.
Montero Pérez O, Sánchez Escudero L, Guzmán Ramos MI, Aviñó Tarazona V. Hypophysitis secondary to pembrolizumab: a case report and review of the literature. Anticancer Drugs. 2022;33(1):94-99. doi:10.1097/CAD.0000000000001129
Kichloo A, Albosta MS, McMahon S, Movsesian K, Wani F, Jamal SM, et al. Pembrolizumab-induced diabetes mellitus pre-senting as diabetic ketoacidosis in a patient with metastatic colonic adenocarcinoma. J Investig Med High Impact Case Rep. 2020;8:2324709620951339. doi: 10.1177/2324709620951339.
Zhao S, et al. Clinical features, diagnosis, and management of pembrolizumab-induced myasthenia gravis. Clin Exp Immunol. 2023;211(2):85-92. doi: 10.1093/cei/uxac108.
Jeroen MK de Filette, Joeri J Pen, Lore Decoster, Thomas Vissers, Bert Bravenboer, Bart J Van der Auwera, Frans K Gorus, Bart O Roep, Sandrine Aspeslagh, Bart Neyns, Brigitte Velkeniers, Aan V Kharagjitsingh, Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review, European Journal of Endocrinology , Volume 181, Issue 3, September 2019, pages 363-374, https://doi.org/10.1530/EJE-19-0291
Joaquim Bellmunt, M.D., Ph.D., Ronald de Wit, M.D., Ph.D., David J. Vaughn, M.D., Yves Fradet, M.D., Jae-Lyun Lee, M.D., Ph.D., Lawrence Fong, M.D., Nicholas J. Vogelzang, M.D. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-1026. DOI: 10.1056/NEJMoa1613683
Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mu-tations. Nat Med. 2014;20(12):1410-1416. doi:10.1038/nm.3746
Paterson AM, Lovitch SB, Sage PT, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212(10):1603-1621. doi:10.1084/JEM.20141030
Girotra M, Hansen A, Farooki A, et al. The Current Understanding of the Endocrine Effects From Immune Checkpoint In-hibitors and Recommendations for Management. JNCI Cancer Spectr. 2018;2(3):1-9. doi:10.1093/jncics/pky021
Iglesias P, Sánchez JC, Díez JJ. Isolated ACTH deficiency induced by cancer immunotherapy: a systematic review. Pituitary. 2021 Aug;24(4):630-643. doi: 10.1007/s11102-021-01141-8. Epub 2021 Mar 24. PMID: 33761049.
Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J Clin Endocrinol Metab. 2014;99(11):4078-4085. doi:10.1210/jc.2014-2306
Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf). 2018;88(2):327-332. doi:10.1111/cen.13483
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J (2017) Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 86:614-620
Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28:583-589
Kichloo A, Albosta MS, McMahon S, Movsesian K, Wani F, Jamal SM, Aljadah M, Singh J. Pembrolizumab-Induced Diabetes Mellitus Presenting as Diabetic Ketoacidosis in a Patient With Metastatic Colonic Adenocarcinoma. J Investig Med High Impact Case Rep. 2020 Jan-Dec;8:2324709620951339. doi: 10.1177/2324709620951339. PMID: 32830561; PMCID: PMC7448133
de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145-156. doi: 10.1055/a-0843-3366
Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, Buffier P, Briet C, Cariou B, Caron P, Chabre O, Chanson P, Cortet C, Do Cao C, Drui D, Haissaguerre M, Hescot S, Illouz F, Kuhn E, Lahlou N, Merlen E, Raverot V, Smati S, Verges B, Borson-Chazot F. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. 2019 Feb;26(2):G1-G18. doi: 10.1530/ERC-18-0320. PMID: 30400055; PMCID: PMC6347286.
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86:614-620.